Glucose Monitors Gone Rogue — Why This Abbott Recall Hits Too Close to Home
I don’t usually write with this much rage and panic, but — wow. The recent news about faulty glucose monitors from Abbott Diabetes Care hits me like a punch in the gut. Because this isn’t some abstract problem. This is real, and it affects families like mine. My 12-year-old daughter has diabetes. So when I saw the recall headlines, I didn’t just read them — I felt them.
Here’s what we know so far — and why you should care, whether you or a loved one uses a continuous glucose monitor (CGM), or if you just care about trusted medical tech being trustworthy.
📉 What’s Happening: The Faulty Sensors, the Recall, the Risks
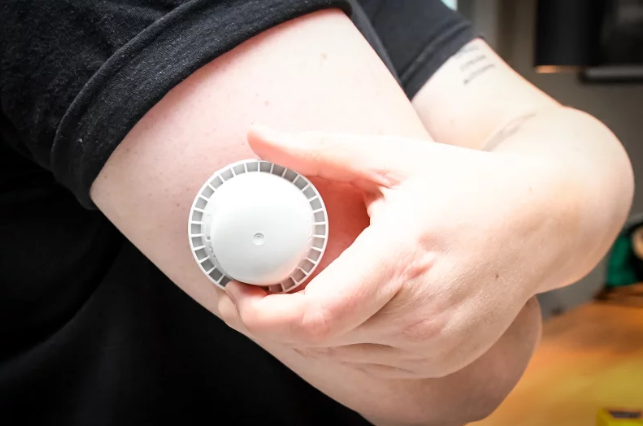
Late in 2025, the U.S. Food and Drug Administration (FDA) issued a public alert regarding certain sensors from Abbott’s CGM line — specifically the FreeStyle Libre 3 and FreeStyle Libre 3 Plus. U.S. Food and Drug Administration+2The Washington Post+2
- The problem stems from one single production line. According to Abbott, internal testing showed that some of these sensors may provide incorrect low glucose readings. U.S. Food and Drug Administration+2WUSF+2
- Why is that dangerous? Because if the CGM thinks your glucose is lower than it is, you might over-correct: eat more carbs than needed, or skip or delay insulin — thinking you’re fine. Over time, that can lead to serious complications. The Washington Post+2ABC News+2
- The scope is massive: about 3 million of these sensors in the U.S. may be affected. That includes both currently in use and unused (or expired) sensors. opb+2WUSF+2
- As of November 14, 2025, Abbott reported 736 serious adverse events globally, and 7 deaths potentially associated with the defect. U.S. Food and Drug Administration+2STAT+2
- In the U.S., there have been 57 injuries tied to the issue (though no confirmed deaths here). The Washington Post+2Yahoo+2
- The affected models include:
- FreeStyle Libre 3: model numbers 72081-01, 72080-01
- FreeStyle Libre 3 Plus: model numbers 78768-01, 78769-01 U.S. Food and Drug Administration+2WBIW+2
Abbott and the FDA are telling users to check their sensor serial numbers — if the device is on the recall list, stop using it immediately and dispose of it. U.S. Food and Drug Administration+2ABC News+2
Abbott says they’ll provide replacements at no cost. opb+1
😡 My Take: This Isn’t a Glitch — It’s a Betrayal
Look, I get it — we love technology. We love being able to glance at a device and know our glucose trends without dealing with finger-pricks every few hours. CGMs like FreeStyle Libre (and others) should be a breakthrough. For my daughter — and kids like her — CGMs can mean fewer interruptions, more freedom, and peace of mind… or so we thought.
But this? This makes me question everything. If a company can ship out millions of devices from a faulty production line — devices that people debate their lives on — then what does “medical device safety” even mean these days?
It’s not enough to say “Sorry, we fixed it now.” Because while they were “fixing it,” real people were harmed. Real families — maybe like yours — were caught in the crossfire.
If your glucose-monitoring relies on a sensor for critical insulin decisions, there’s no margin for error. This isn’t “inconvenient bug.” This is a potential death sentence.
✅ What You Should Do Right Now (If You Use a CGM) — Because This Is Serious

If you or someone you love uses a FreeStyle Libre 3 or 3 Plus CGM:
- Check the model number / serial number — go to the official recall-check site (as directed by Abbott/FDA) and verify if your sensor is one of the affected units. U.S. Food and Drug Administration+1
- If your sensor is on the recall list, stop using it — immediately. Dispose of it. Don’t wait for “maybe.” U.S. Food and Drug Administration+2opb+2
- Request a free replacement from Abbott — the company has said they will replace affected sensors at no cost. opb+1
- Use a backup glucose-monitoring method until you get a replacement — a finger-stick meter, or other CGM not affected. Do not trust readings from a sensor you suspect might be faulty. The Washington Post+1
- Watch symptoms, not just numbers — especially low-glucose symptoms. If you feel “off,” treat accordingly even if the sensor seems fine. In other words: treat the person, not just the number.
If you’re a parent of a diabetic child — like me — add one more step: maybe hug them a little tighter. Double-check their gear. Because when lives are involved… trust is mandatory.
🔍 What This Means for the Future — Accountability, Oversight, and Trust
This isn’t just about faulty sensors. It’s a wake-up call about how we treat medical devices in 2025. A few takeaways that, as a parent and as someone who writes about real-world risk, I think deserve serious attention:
- Quality control can’t be optional. When a “single production line error” can result in hundreds of injuries and deaths, we can’t treat it as a minor oversight. Medical device manufacturers need stricter checks — especially for something as widespread and critical as CGMs.
- Transparency matters — batch numbers, serial numbers, impacted lots — this needs to be clearly and publicly available. Not buried in fine print or behind layers of website navigation. Families deserve straight-up clarity.
- Backup systems need to be standard. We can’t go into the future thinking “everything will work flawlessly.” Medical technology is powerful — but human lives are fragile. There must always be redundant safety nets.
- Regulatory bodies must hold devices accountable. If a device fails at its core function (giving accurate glucose readings), it should not be on the market. Period.
- User empowerment through information. Patients — especially parents, caregivers — deserve clear, simple, timely instructions when issues arise. Because when your child’s health is on the line, you shouldn’t need a medical degree to know what to do.
💔 My Personal Warning (Because It’s Real for Me — and Could Be for You)
As I type this, I’m thinking about my daughter. A kid who deserves to run, play, grow up — not worry about whether the device on her arm tonight lies to both of us about something as fundamental as blood sugar.
When I put her to bed, I want to sleep easy. I want to trust that the monitor on her skin — a piece of tech we all believed in — is doing its job. But now? That trust is shaken.
So I’m going to be over-the-top paranoid. I’m going to triple-check serial numbers. I’m going to treat finger-stick meters like ancient relics — but also like lifelines. I’m going to read every food label, count every carb, watch every insulin dose. I’m going to stay alert. Because this isn’t a “maybe” — this is real.
If that makes me “that overprotective parent”? Good. I’ll wear that badge proudly.
If you’re reading this and your loved one depends on a CGM: please don’t brush this off. Please don’t assume “it’s probably fine.” Check. Verify. Act.
Because the alternative is unthinkable.
✅ FAQ: Abbott Glucose Monitor Recall — What You Need to Know
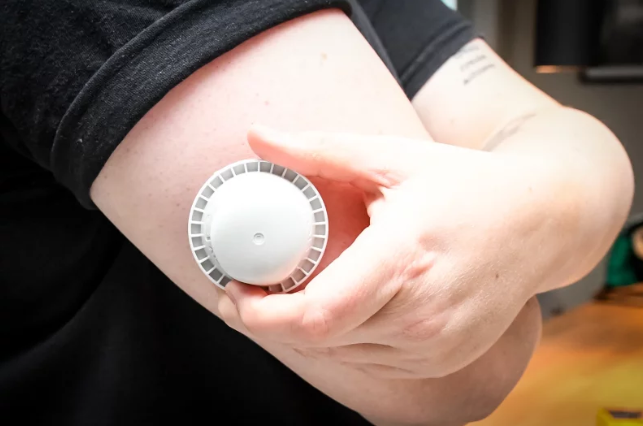
❓ Which glucose monitors are part of the recall?
The recall affects specific units of the FreeStyle Libre 3 and FreeStyle Libre 3 Plus continuous glucose monitor (CGM) sensors. Only certain sensors from a faulty production line are impacted, and each device must be verified by serial number.
❓ What exactly is wrong with the sensors?
Some sensors may report incorrectly low glucose readings.
This can cause people to:
- Eat unnecessary carbohydrates
- Delay or skip insulin
- Mismanage their glucose levels
- End up in the ER or worse
Incorrect low readings can be extremely dangerous — especially for insulin-dependent diabetics.
❓ How many people have been affected?
According to FDA reports:
- 7 deaths may be linked to defective sensors
- 736 serious injuries reported globally
- Tens of millions of sensors are in circulation
- Roughly 3 million affected devices were distributed in the U.S. alone
❓ How do I know if my sensor is affected?
You must check your sensor’s serial number on Abbott’s official recall-check page or through FDA guidance. Only specific lots are impacted.
If yours is affected: stop using it immediately, dispose of it safely, and request a replacement.
❓ What should I do if my device is on the recall list?
- Stop using the sensor immediately
- Switch to a backup (finger-stick meter or another CGM)
- Request a free replacement from Abbott
- Monitor symptoms, not just the numbers
- Contact your doctor if you feel unsafe or confused about your readings
❓ Are replacements free?
Yes. Abbott has stated they will replace affected sensors at no cost, including shipping.
❓ Are Libre 1 or Libre 2 sensors affected?
No — the recall and safety warning apply only to certain Libre 3 and Libre 3 Plus sensors.
❓ Should I panic?
No — but you should take it seriously.
This recall is a big deal, especially for diabetics who rely heavily on CGMs for safe insulin decisions. Your best move is to check your device’s serial number, stay informed, and always have a backup monitoring method.
👉 Before you go — do yourself a favor and smash that Linktr.ee button like it’s a faulty glucose sensor.
If you want quick, painless access to all my chaos —
my X, my TikTok, and my YouTube —
hit my Linktr.ee below:
➡️ Click here for instant access
Because let’s be honest — keeping up with my content is way less dangerous than relying on a sketchy CGM.
Stay informed. Stay sarcastic. Stay alive.
And click the damn link.